Surface Tension Is Same for Different Liquids Explain
Surface tensions of several liquids are presented in Table 2. Despite the use of the prefix omni- 6 1518 however no natural or man-made surface has been reported to repel liquids of extremely low surface tension or energy ie γ 15 mJm 2 such as fluorinated solvents which completely wet existing materials 10 1921.
Surface Tension Surface Tension Of Liquids Surface Tension In Soap Bubble
Surface tension is an essential property of liquids needed for the theoretical and practical studies of different processes such as for instance bubble and droplet formation wetting capillarity detergency atomization formation of aerosols and sprays injection of.

. Among common liquids water exhibits a distinctly high surface tension due to strong hydrogen bonding between its molecules. An open question with great relevance for industrial production processes. Why do large gas bubbles in viscoelastic liquids such as polymer and protein solutions rise so much faster than expected.
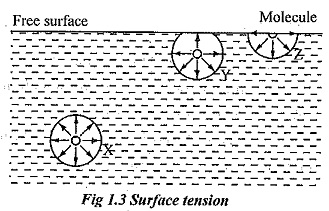
As a result of this high surface tension the surface of water represents a relatively tough skin that can withstand considerable force without breaking.
Why Is There Surface Tension Only On The Top Of Water And Not Inside It Quora

Surface Tension Of Various Polar And Apolar Liquids Download Table

No comments for "Surface Tension Is Same for Different Liquids Explain"
Post a Comment